The Research Preparedness Program for East and Central Africa (RPECA) is a multi-region, multi-country initiative led by PATH as the initial Technical Coordinating Partner (TCP), in collaboration with CEPI, Africa Centers for Disease Control and Prevention (CDC), and regional stakeholders. RPECA is designed to strengthen regional clinical research ecosystems to catalyze the ability to efficiently and effectively conduct Good Clinical Practice-compliant late-stage vaccine trials and to support the rapid generation of emergency evidence in the context of infectious disease outbreaks.
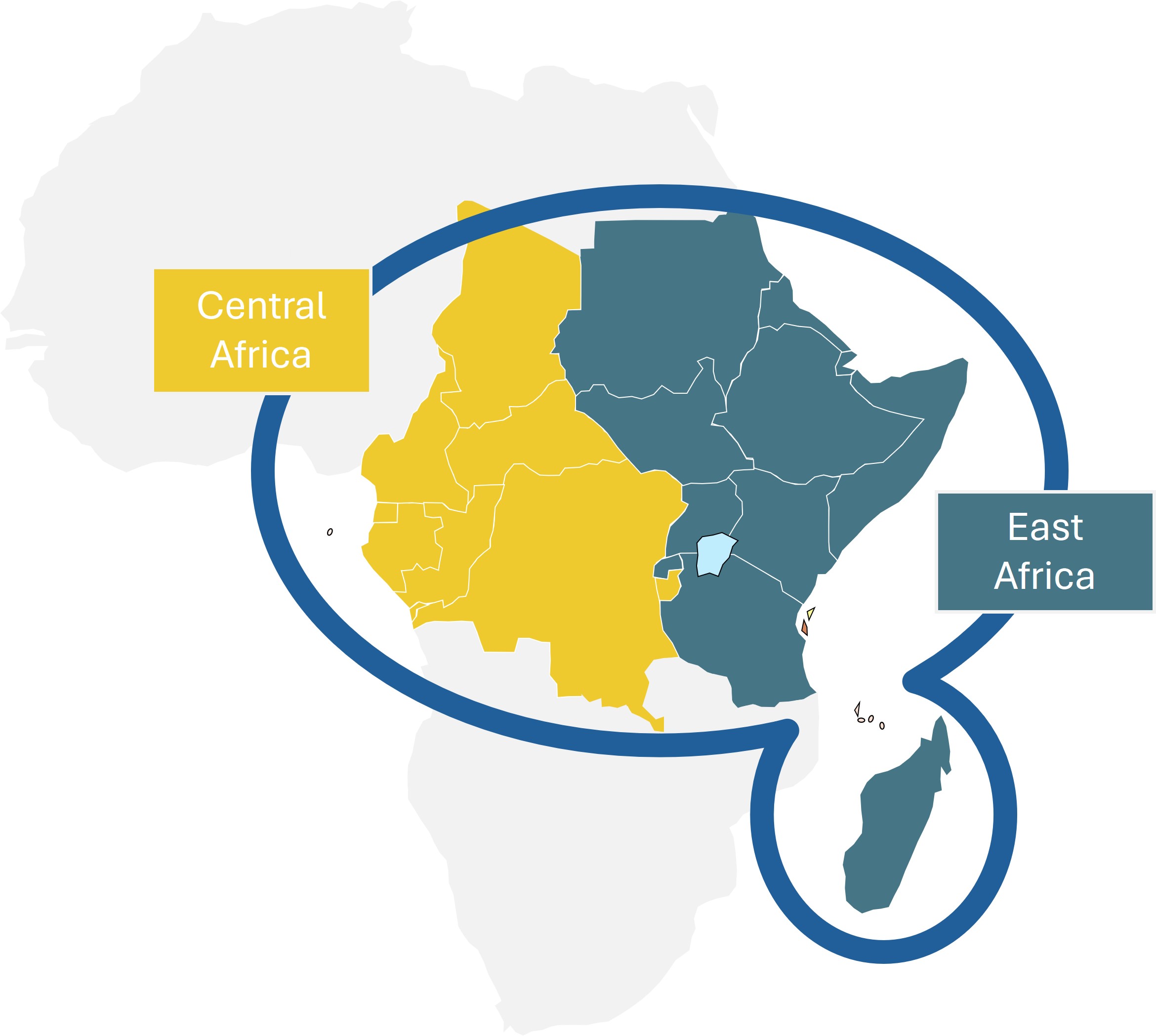 Why East and Central Africa? Why East and Central Africa?
East and Central Africa face a high risk of emerging and re-emerging infectious diseases such as mpox, Rift Valley Fever, and filoviruses. Despite past investments in vaccine development, many clinical research entities lack sustained readiness for generating emergency evidence (including clinical trials and real-world evidence [RWE]) during infectious disease outbreaks. RPECA addresses this gap by supporting preparedness to conduct research as part of outbreak response and by maintaining a sustainable and “ready” outbreak response network through preparedness to conduct research for routine vaccine development.
Technical Coordinating Partner (TCP)
PATH is a global nonprofit organization dedicated to achieving health equity. It develops and scales innovative solutions—spanning science, technology, advocacy, and partnerships—to address the world’s most pressing health challenges. As part of establishing RPECA as a longer-term research preparedness program and in alignment with key stakeholder goals and country priorities, PATH will be leading the identification and selection of an Africa-based co-TCP to ensure regional ownership and sustainability.
Program Structure
Anchored in continental, regional, and local stakeholder engagement, RPECA aims to build frameworks for rapidly generating routine and emergency evidence during outbreaks of CEPI priority diseases, such as mpox, Rift Valley Fever, and filoviruses. It focuses on supporting clinical trial site, institutional, and broad research ecosystem readiness via pursuit of a “hub-and-spoke” model, including regulatory and ethics committee engagement. RPECA builds on lessons from the International Vaccine Institute and Medical Research Council Unit The Gambia who co-lead the TCP for West Africa. The following initial activities are currently planned or ongoing:
- A comprehensive stakeholder mapping exercise is underway. This mapping will identify, categorize, and engage key actors across East and Central Africa who are essential to advancing clinical research preparedness for both routine and emergency vaccine trials. It combines desk research and interviews to assess policy landscapes, identify advocacy entry points, and build country-specific stakeholder lists, with stakeholders categorized by type, region, specialization, and funding sources.
- A regional stakeholder convening is planned for the fourth quarter of 2025 to align regional priorities, expand on the stakeholder mapping, contribute towards the development of a regional research preparedness roadmap, and integrate with broader strategic initiatives such as those led by Africa CDC.
- Providing technical assistance for a cohort event monitoring (CEM) post-vaccination mpox safety study in the Democratic Republic of Congo (DRC) that is being led and implemented by the Africa CDC and the DRC’s National Centre for Pharmacovigilance and Expanded Programme on Immunization. The CEM study of adverse events following mpox immunization protocol aims to strengthen regional capacity for rapid, high-quality evidence generation on vaccine safety during an ongoing mpox outbreak and is based on a request for assistance from the DRC-led protocol team. Activities include providing overall study management advice and country-specific mpox vaccine rollout intelligence, as well as developing operational, data management, and statistical analysis plans and associated training materials to support local readiness.
RPECA partners include:
 Africa CDC is a continental autonomous health agency of the African Union. Its mission is to strengthen the capacity and capability of Africa’s public health institutions and partnerships to detect, prevent, control, and respond quickly and effectively to disease threats and outbreaks. This is achieved through evidence-based policies, programs, and interventions. Africa CDC is a continental autonomous health agency of the African Union. Its mission is to strengthen the capacity and capability of Africa’s public health institutions and partnerships to detect, prevent, control, and respond quickly and effectively to disease threats and outbreaks. This is achieved through evidence-based policies, programs, and interventions.
 IQVIA is supporting CEPI as a global service provider and provides technical expertise to CEPI's partners. IQVIA is a global leader in health information technologies, clinical research, and data analytics. IQVIA provides services and solutions that advance medical research, improve patient care, and drive healthcare innovation. IQVIA is supporting CEPI as a global service provider and provides technical expertise to CEPI's partners. IQVIA is a global leader in health information technologies, clinical research, and data analytics. IQVIA provides services and solutions that advance medical research, improve patient care, and drive healthcare innovation.
RPECA will also actively engage with:
- Intra- and pan-African entities
- Professional organizations
- Ministries of health, national regulatory authorities, and ethics committees
- Academic institutions and clinical trial networks and sites
- World Health Organization’s Regional Office for Africa
|

 Why East and Central Africa?
Why East and Central Africa?