The largest-ever Lassa fever research programme in West AfricaBackgroundLassa fever, a zoonotic acute viral haemorrhagic disease caused by the Lassa virus, has persisted as an endemic threat in Western Africa since its discovery in 1969. While most cases are asymptomatic or mild, severe cases require hospitalisation and often result in death due to organ failure. Survivors may suffer from permanent hearing issues, known as sensorineural hearing loss (SNHL). Current primary treatments for patients involve early supportive care (e.g. fluids & pain relief) and the administration of the anti-viral drug ribavirin; however, the effectiveness of the latter remains debated. Therefore, the need persists for a tailored preventive intervention against Lassa fever infection – leading to the pursuit of an effective vaccine.
Enable 1.0
|
Enable 1.5 Mid-Term Site Visits - Progress and InsightsIn June 2025, CEPI's Enable 1.5 team, in collaboration with MMARCRO, IVI, and MRC Gambia, conducted mid-term site visits across the three participating countries. The valuable field trip began in Kenema, Sierra Leone, continuing through to Phebe, Liberia, and concluding in Abuja, Nigeria. All sites are progressing with the study as expected. Discussions highlighted the crucial role of sustained and enhanced community engagement to mitigate participant attrition, as well as continuous communication regarding the impact of Enable’s capacity-strengthening efforts.
In October 2025, CEPI and partners (IVI, NCDC, Epicentre and MMARCRO) visited Enable 1.5 sites in Edo, Ondo, and Abakaliki States in Nigeria. The team reviewed scientific progress and deliverables, while also evaluating laboratory and clinical infrastructure, and troubleshooting as needed. Such visits strengthen collaboration among PIs, sites, and partners, and improve adherence to study timelines to guarantee the success of Enable 1.5 in generating key data for Lassa virus vaccine trials in the region.
|
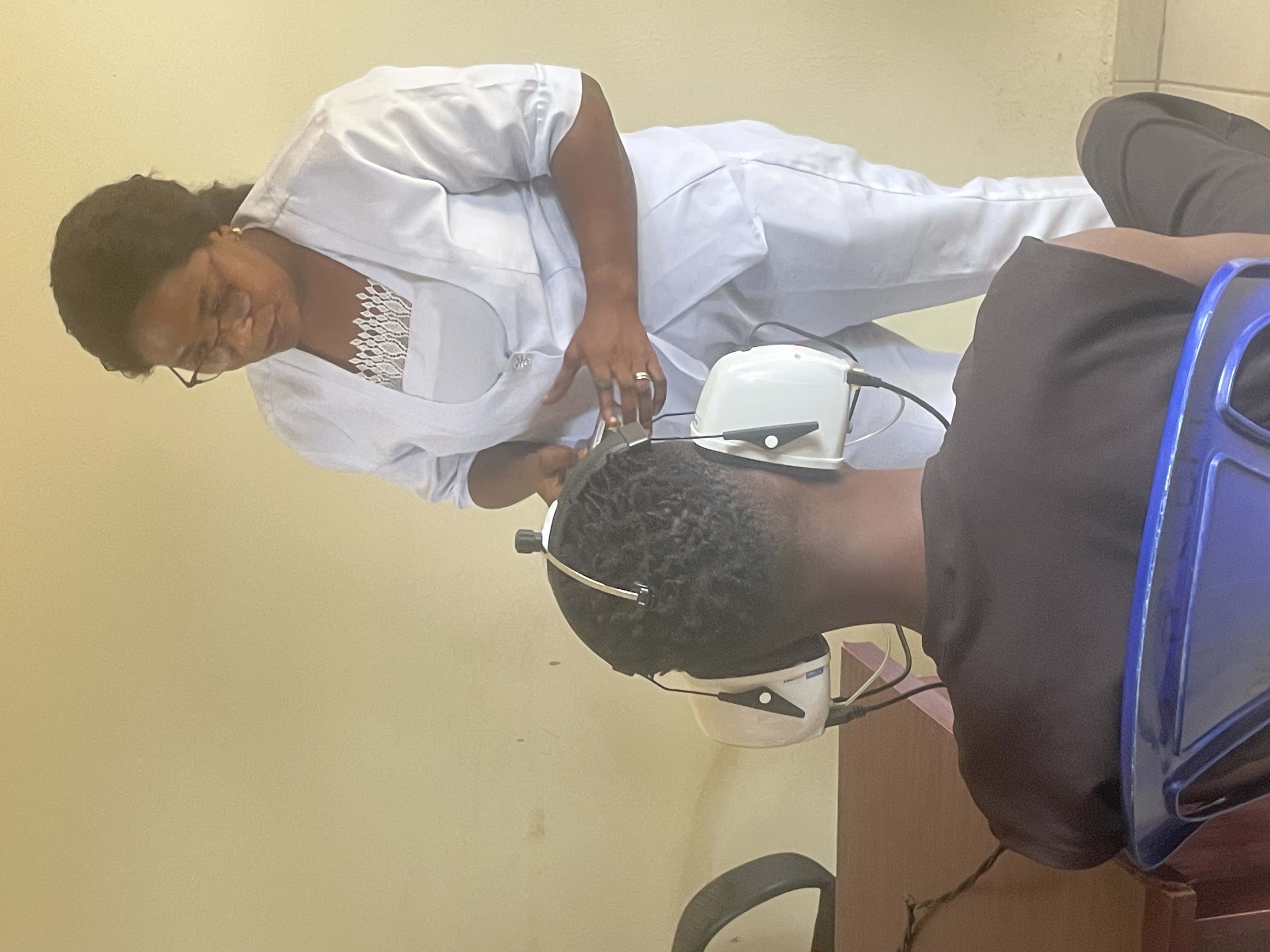
Sensorineural hearing loss assessmentInfection with the Lassa virus may result in sensorineural hearing loss (SNHL), which can affect survivors for a lifetime. Understanding the baseline level of SNHL in communities where CEPI is studying Lassa fever is key, as well as ascertaining the incidence of new SNHL cases due to new Lassa virus infections. As part of Enable 1.5, CEPI has provided KUDUwave audiometers with laptops, and newly trained staff are testing participants' hearing.
|
Resources and PublicationsProspective cohort study to evaluate Lassa fever incidence, symptoms and coinfection with malaria in West Africa: the Enable Lassa Research Programme (‘ENABLE 1.5’) – study protocol | By Mandi H, Asogun D, Ayodeji O, et al. | BMJ Public Health 3:e001960 (July) 2025From the pain of Ebola to the passion to fight Lassa - an interview with Dr Donald Grant, Sierra Leone Ministry of HealthA deadly viral illness is exploding in West Africa. Researchers are scrambling to figure out why Science | Vol 383, Issue 6685Events Incidence of Lassa fever disease and Lassa Virus infection in five West African countries: a prospective, multi-site, cohort study presentation at the American Society of Tropical Medicine and Hygiene Conference by Professor Danny Asogun | New Orleans on 16 November 2024The ENABLE Lassa research programme: addressing key epidemiology gaps for Lassa fever vaccine development - a symposium at the World Congress of Epidemiology (25 September 2024, Cape Town) Programme | World Congress of Epidemiology 2024 (wce2024.org)Official Launch of Enable 1.5 in Nigeria in early October 2024. Evidence generated by the Enable Programme will be critical for the upcoming Lassa fever phase III vaccine trials:
|
 In Enable 1.0 over 23,000 participants were followed up in 2019–2023 to estimate the incidence of Lassa fever infection and disease across five West African countries (Benin, Guinea, Liberia, Nigeria, and Sierra Leone). Preliminary results indicate that Lassa fever baseline seroprevalence — a measure of the proportion of people who have antibodies against Lassa virus at a given time, indicating previous exposure — was around 30%, ranging from 2% in Benin to 48% in Liberia. During the study, 39 cases of symptomatic Lassa fever were observed. The results of this study are soon to be published.
In Enable 1.0 over 23,000 participants were followed up in 2019–2023 to estimate the incidence of Lassa fever infection and disease across five West African countries (Benin, Guinea, Liberia, Nigeria, and Sierra Leone). Preliminary results indicate that Lassa fever baseline seroprevalence — a measure of the proportion of people who have antibodies against Lassa virus at a given time, indicating previous exposure — was around 30%, ranging from 2% in Benin to 48% in Liberia. During the study, 39 cases of symptomatic Lassa fever were observed. The results of this study are soon to be published.