
IntroductionComparing immune responses against different potential vaccine candidates is challenging. Biological variation and technical differences (how and where specimens are collected, transported, stored, and analyzed) impacts the quality and usefulness of the data produced and makes comparisons between measurements in individual laboratories difficult. Launched in 2020 in response to the COVID-19 pandemic, CEPI’s Centralised Laboratory Network (CLN) is the world’s largest network of vaccine testing laboratories.
|
The network laboratories
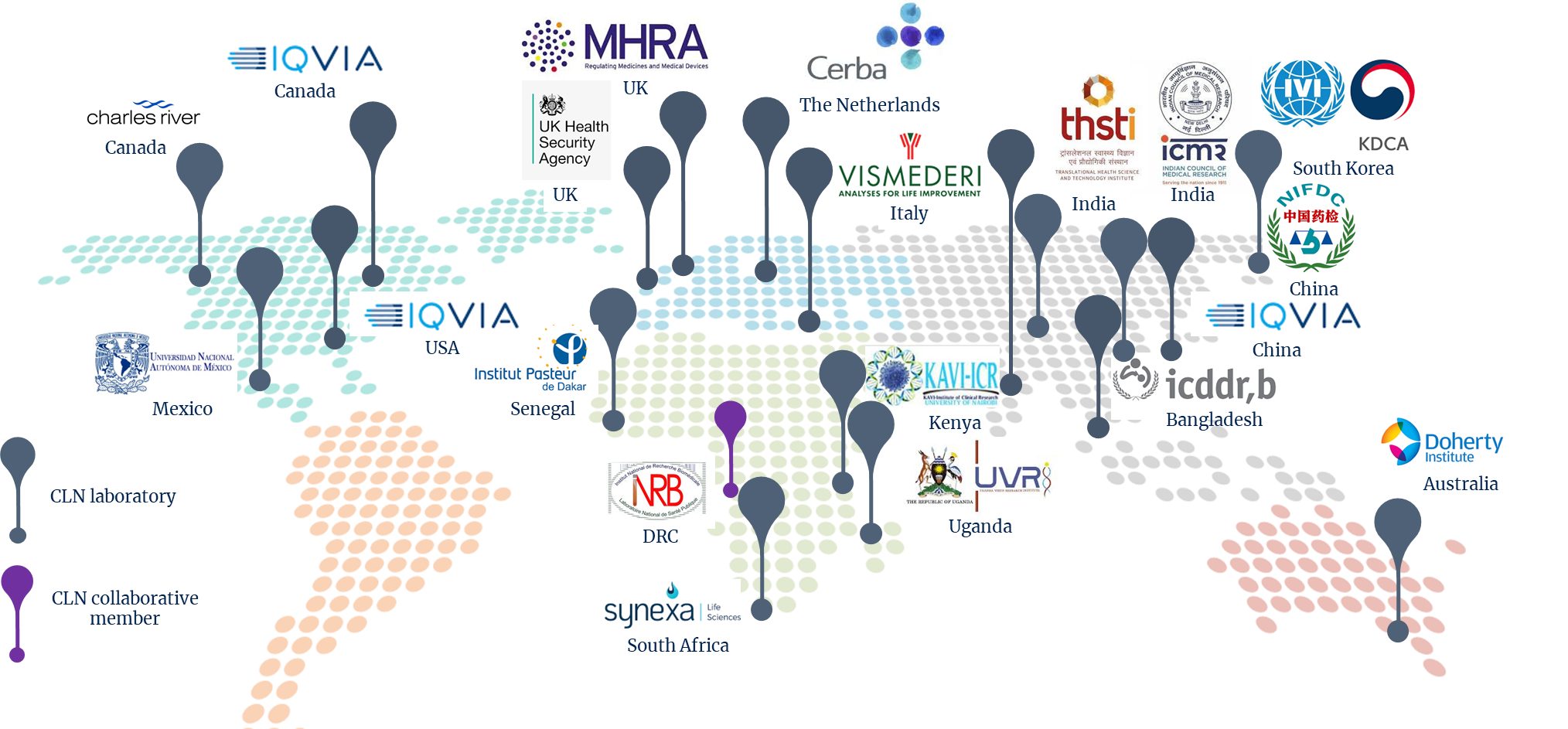
|
Laboratory |
Location |
Type of institution |
Disease Experience |
Contracted to work on |
|
UKHSA |
UK |
Govt. Institute |
SARS, Lassa, Nipah ,MERS, CCHF, Zika, Hantavirus, Influenza, Monkeypox virus, Ebola, Marburg |
SARS-CoV-2 and new pathogens |
|
MHRA |
UK |
Govt. Institute |
Lassa, Nipah, RVF, MERS, Chick, Ebola, Zika, CCHF |
SARS-CoV-2 and new pathogens |
|
Nexelis, Q2 Solutions |
Canada |
Private |
SARS, MERS, Chik, Nipah, Ebola |
SARS-CoV-2 and new pathogens |
|
VisMederi |
Italy |
Private |
SARS, Lassa, Influenza, Yellow Fever |
SARS-CoV-2 and new pathogens |
|
Viroclinics |
Netherland |
Private |
SARS, MERS, Influenza, RSV, Polio, Measles, Rabies, Deng |
SARS-CoV-2 and new pathogens |
|
THSTI |
India |
Govt. Institute |
SARS-CoV-2, Various Bacteria infections |
SARS-CoV-2 |
|
ICMR |
India |
Govt. Institute |
SARS, Chick, Dengue, Japanese encephalitis, CCHF, Nipah, Zika, Ebola |
New pathogens |
|
Icddr,b |
Bangladesh |
Non-for profit |
Influenza, Polio, Mumps, Measles, Rubella, Hepatitis B, SARS, Nipah |
SARS-CoV-2 and new pathogens |
|
Q2 Solutions |
China |
Private |
SARS, HIV |
SARS-CoV-2 and new pathogens |
|
Synexa |
South Africa |
Private |
SARS, HIV, TB, Malaria, Influenza, Hepatitis, CMV, EBV |
SARS-CoV-2 and new pathogens |
|
KAVI |
Kenya |
Non-for profit |
SARS, Chikungunya, Lassa, MERS, HIV, DENGUE RVF |
SARS-CoV-2 and new pathogens |
|
IPD |
Senegal |
Non-for profit |
SARS, RVF, Ebola, Chikc, Lassa, Ebola, Yellow fever, Marburg |
SARS-CoV-2 and new pathogens |
|
UVRI |
Uganda |
Non-for profit |
SARS, HIV, Ebola, Yellow Fever, RVF |
SARS-CoV-2 and new pathogens |
|
UNAM |
Mexico |
Non-for profit |
Ebola, Dengue, Zika, Rotavirus, Influenza |
SARS-CoV-2 and new pathogens |
|
Charles River |
USA |
Private |
CMV, Adenovirus, HIV, SARS-CoV2, HepB, VZV, Malaria, Dengue, Ebola |
New pathogens |
|
Doherty |
Australia |
Non-for profit |
HIV, influenza, SARS, Paxamyxovirus (Nipah, Hendra), Chick, Ebola, MERS |
SARS-CoV-2 and new pathogens |
|
Q2Solutions |
USA |
Private |
SARS-CoV-2 S ELISA |
SARS-CoV-2 and new pathogens |
|
NIFDC |
China |
Govt. Institute |
SARS-Cov-2, HPV, MERS-CoV, SARS-CoV, LASV, Ebola, Marburg, RVF, Nipah, SFTS, CHIKF |
New pathogens |
|
INRB |
DRC |
Govt. Institute |
Ebola, Polio, Influenza, Mpox, Measles, Rotavirus |
New pathogens |
|
IVI |
South Korea |
Non-for-profit |
Coronaviruses, Chikungunya, Lassa, Influenza, Hep E, HPV |
|
|
KDCA |
South Korea |
Govt.Institute |
Coronaviruses, Chikungunya, Lassa, Influenza, Hep E, HPV |
The Centralized Laboratory Network in numbers
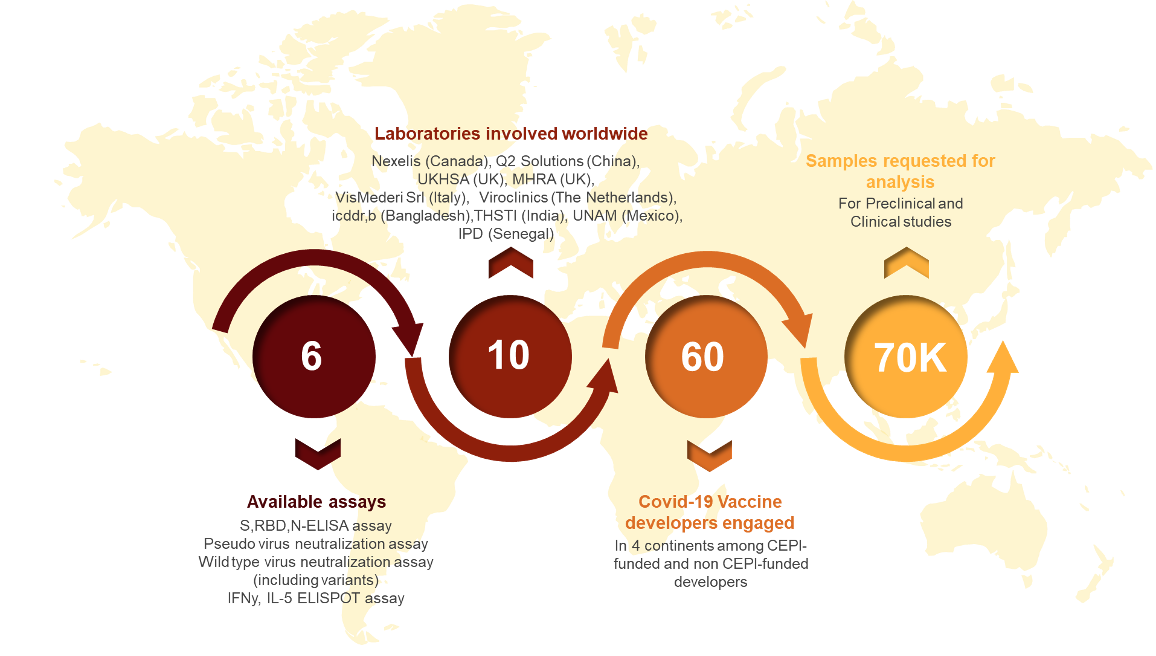
Global CLN Advisory Council (GCAC) at CEPI's Centralised Laboratory NetworkCEPI's Centralised Laboratory Network (CLN) is pleased to announce the formation of the Global CLN Advisory Council (GCAC), which aims to ensure that our 18 facilities within the network are more actively involved in critical decision-making processes. This initiative will offer a unique opportunity to address both current and future challenges within the CLN. The GCAC members will play a key role in:
After a competitive selection process, the following individuals have been chosen to represent various facilities on the GCAC:Luc Gagnon - Nexelis-IQVIA
|
PublicationsPublications and other resources from the CLN are available here: Publications |