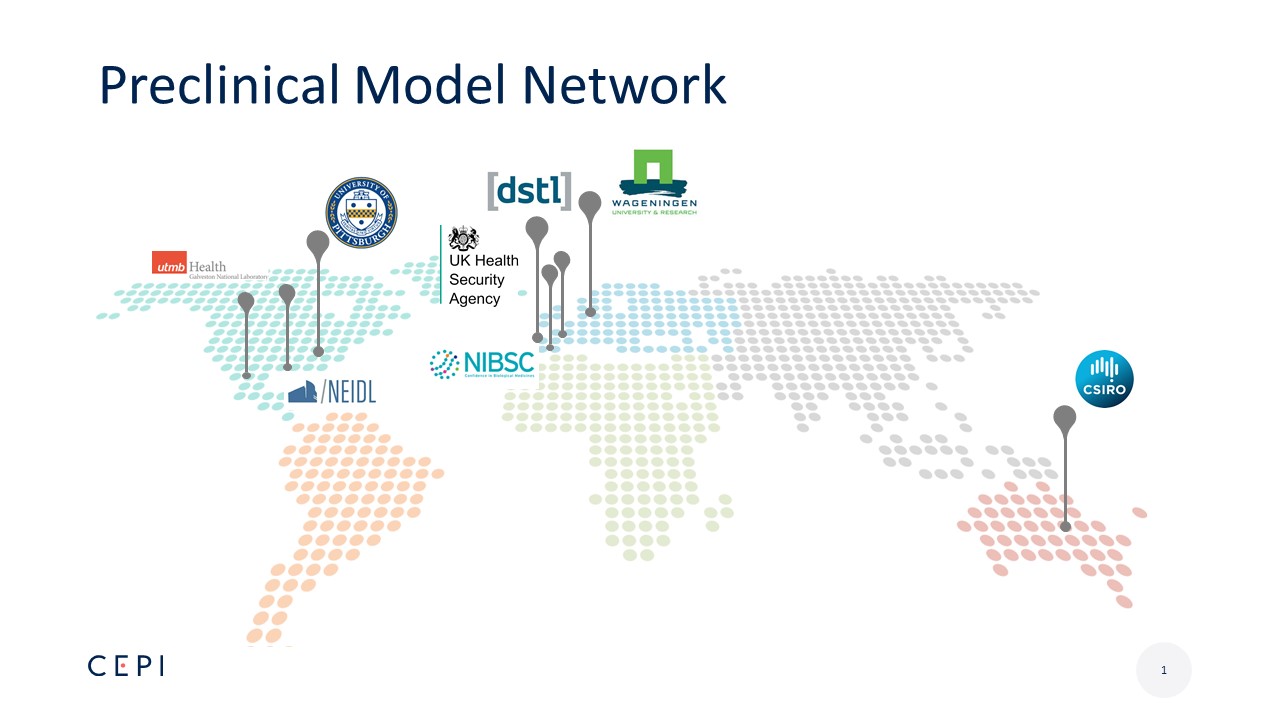
Preclinical Model Network
About the network
- Preclinical testing network of partners with capability to handle BSL3 and/or 4 pathogens, and experience with CEPI priority pathogens: MERS, Nipah, Rift Valley Fever, Chikungunya and Lassa viruses, now also SARS-COV-2
- Includes partners who can perform high quality studies under a rigorous quality system. Research output must meet GLP or high-quality standards to generate data packages supporting submission to regulatory agencies for vaccine product development
- Perform work in alignment with UK NC3Rs guidelines. CEPI’s funding sources require CEPI to evaluate compliance with high animal ethics standards
- CEPI’s laboratory partners are located in Europe, US and Australia
- Currently testing/developing models for COVID-19 infection
- Where models are in place, preclinical vaccine testing for CEPI vaccine development partners is underway
Articles
1. Publications of model work
UK HSA:
CSIRO
WHO
2. Publications of vaccine testing studies
Other Texts
CEPI’s policy
