CEPI Centralized Laboratory NetworkOverview The Coalition for Epidemic Preparedness Innovations (CEPI) is supporting different vaccine candidates targeting the SARS-CoV-2 virus (Severe acute respiratory syndrome coronavirus 2). Comparing immune responses against different vaccine candidates intended for the prevention of SARS-CoV-2 is challenging. In order to reduce some of this complexity, better assess the immunological profile of each vaccine candidate, and provide robust assays for regulatory purposes, CEPI created a Centralized Laboratory Network to test the immune response elicited by different vaccine in preclinical studies and Phase I and IIa clinical studies. Objective The CEPI Centralized Laboratory Network is open to all COVAX funded and non-funded vaccine developers:
All COVID-19 vaccine developers are invited to apply to use the Network.
|
Qualified assays available:
More information on processing of samples, shipment and data handling within the CEPI Centralized Laboratory Network can be found here. The testing service is free of charge, except for the shipment costs. To check your eligibility, complete the Sample Analysis Request Form. |
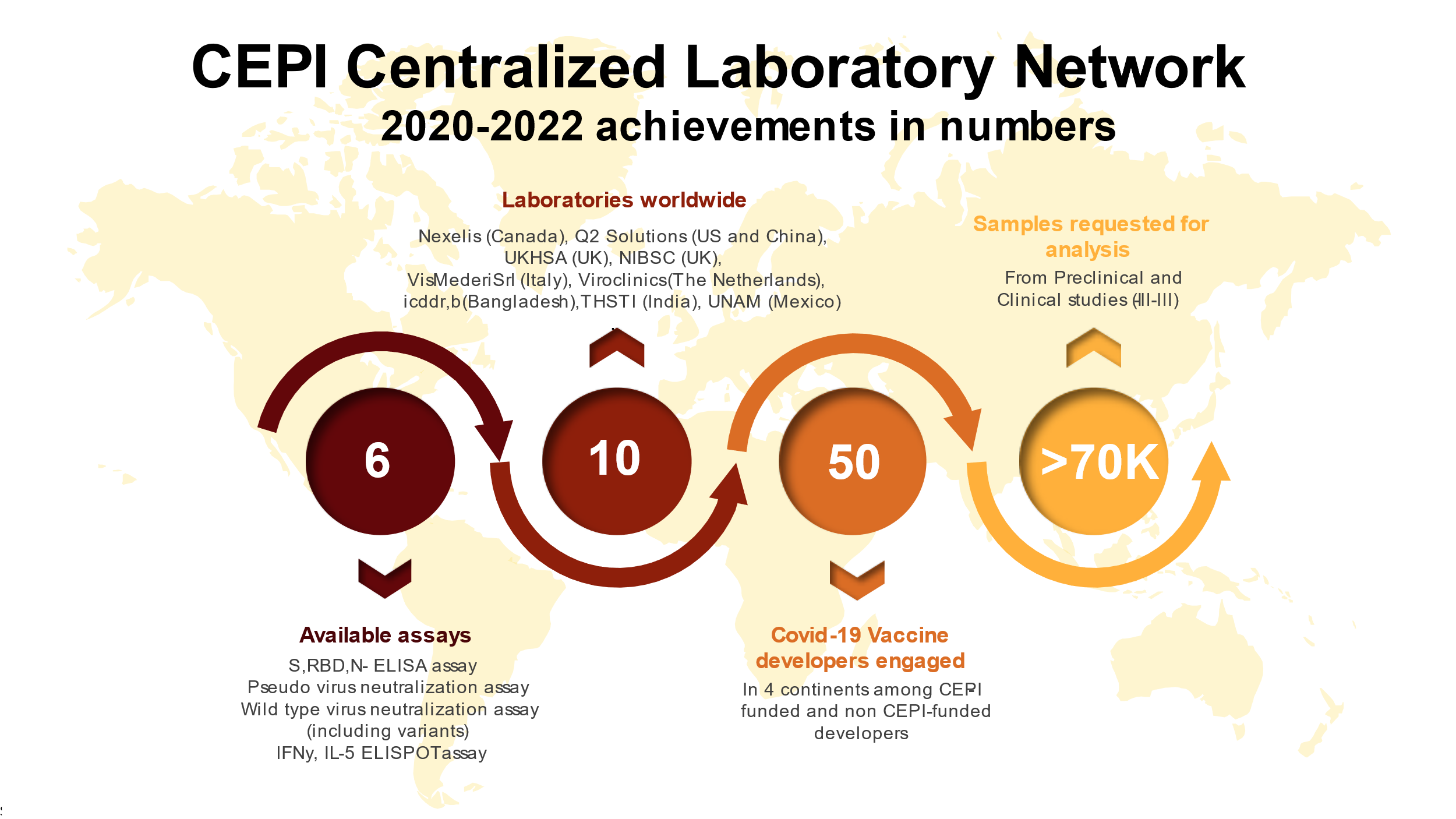
The Centralized Laboratory Network in numbers:
Workshops, Conferences, Congresses, WebinarsExpanding the CEPI Centralized Laboratory Network - Webinar for the labs | 7 - 9 December 2021 | Presentation Material | Video Recording | Q&A | Transcript (in English) Webinar 'Standardization of Immune Response Assays to COVID-19 Vaccines: A Year’s Experience Transferring Assays to a Global Network of Labs | 31 August 2021 | Presentation Material | Report | Recording Part 1 - Lessons Learned | Recording Part 2 - The Future | Webinar on the Centralized Laboratory Network | 12 March 2021 | Presentation Material | Recording Workshop on COVID-19 Correlates of Protection | COVAX Clinical Development SWAT Team | 19 Nov 2020 | Workshop Full Report |
Publications
|

 The network laboratories:
The network laboratories: